Lab Chip, 2010 | DOI: 10.1039/b917486a | Paper
(http://www.rsc.org/publishing/journals/LC/article.asp?doi=B917486A)
Pouya Rezai
a,
Asad Siddiqui
b,
Ponnambalam Ravi Selvaganapathy
*a and
Bhagwati P. Gupta
*b
aDepartment of Mechanical Engineering, McMaster University, JHE-212/B, 1280 Main Street West, Hamilton, ON, Canada L8S 4K1. E-mail: selvaga@mcmaster.ca; Fax: +1 905 572 7944; Tel: +1 905 525 9140 ext. 27435
bDepartment of Biology, LSB-531, McMaster University, 1280 Main Street West, Hamilton, ON, Canada L8S 4K1. E-mail: guptab@mcmaster.ca
The nematode (worm) Caenorhabditis elegans is one of the most widely studied organisms for biomedical research. Currently, C. elegans assays are performed either on petri dishes, 96-well plates or using pneumatically controlled microfluidic devices. In this work, we demonstrate that the electric field can be used as a powerful stimulus to control movement of worms in a microfluidic environment. We found that this response (termed electrotaxis) is directional, fully penetrant and highly sensitive. The characterization of electrotaxis revealed that it is mediated by neuronal activity that varies with the age and size of animals. Although the speed of swimming is unaffected by changes in the electric field strength and direction, our results show that each developmental stage responds to a specific range of electric field with a specific speed. Finally, we provide evidence that the exposure to the electric field has no discernible effect on the ability of animals to survive and reproduce. Our method has potential in precisely controlling, directing, and transporting worms in an efficient and automated manner. This opens up significant possibilities for high-throughput screening of C. elegans for drug discovery and other applications.
The objective of the majority of biological research today is to understand human diseases and develop new and effective treatments for them. Multidisciplinary research approaches serve to identify the genetic basis of these pathologies in order to better understand and treat their mechanism of action. Due to the enormous complexity of cellular and molecular processes, as well as ethical issues associated with experiments on human subjects, researchers have focused on a number of eukaryotic systems that are simpler and easier to manipulate, yet complex enough to address many of the questions relevant to human biology. The nematode Caenorhabditis elegans (C. elegans) is one such model organism that has greatly facilitated the study of conserved biological processes. It offers a number of useful features such as small size (~1000 somatic cells), well-mapped neuronal connectivity,1 transparency, short life cycle (~2.5 days), and the ability to generate many progeny in a relatively short time. Furthermore, the identity and lineage of every cell in the worm is known which enables researchers to address biological questions at single cell resolution. The amenability of the organism to genetic analysis has led to isolation of a large pool of mutant strains (see http://www.wormbase.org) that can be effectively used to study gene function in regulating various developmental, behavioral, and physiological processes. Such studies have established the roles of many genes (e.g., HOX family members) and signaling pathways (e.g., Ras) in normal and disease processes,2,3 thereby facilitating the study of their homologues in other organisms including humans.
The analysis of the C. elegans genome sequence has revealed the presence of a large number (~65%) of human disease orthologs4,5 that are very useful in investigating the underlying mechanisms of gene function. Worms have been used successfully as models for a variety of human disorders such as obesity,6 hypertension,7 Duchenne's muscular dystrophy (DMD),8 and neurodegeneration (e.g., Huntington's disease, HD; Parkinson's disease, PD).9–11 Researchers have established mutant strains for these diseases that serve as models to study the underlying mechanism as well as to search for chemical compounds/drugs to inhibit the defects. For example, in the case of the C. elegans HD model, chemical screening has identified two compounds, mithramycin (MTR) and trichostatin A (TSA), that have significant effect on promoting neuronal survival.10
Among the various features of C. elegans, its small size and the ability to grow in liquid media have facilitated high-throughput screenings (HTS) for chemicals. These chemicals can affect physiological processes and may serve as potential drug candidates for a variety of medical applications.12–14 Traditional methods of drug screening using C. elegans involve exposure of animals to chemicals/drugs in culture plates (e.g., 96-well plate) while monitoring subsequent effects on growth, movement and other processes by visual inspection.8 This method is time-consuming, expensive, tedious and prone to subjectivity. Recently, microfluidic devices have been used to facilitate more precise and quantitative studies in C. elegans.15,16 Because of their pneumatic actuation these devices are useful in transporting and immobilizing animals for various purposes. These include culturing,17 sorting and screening,18,19 and in vivo analysis of neuronal activity and regeneration.20–26
We wanted to develop a new microfluidic device that, unlike pressure-driven control, uses a stimulus to manipulate animal movement. Here we show that electrical signal can act as a powerful stimulus to precisely control and direct movement of C. elegans in microchannels. Worms respond to diverse external stimuli such as light, temperature, chemicals, electric and magnetic fields and exhibit either attractive (e.g., food, electrical) or repulsive (e.g., high temperature) responses.27 The response of worms to an electric field stimulus was examined in some detail.28–30 Although its biological relevance remains unclear, it has been suggested that the ability of C. elegans to respond to electrical signal may have evolved as a host finding cue in parasitic nematodes. The genetic analysis of electric field response in C. elegans has revealed that certain sensory neurons mediate attraction towards the cathode.29 However, since these experiments were performed on open gel plates that are typically used to culture the organism, the electrical conditions were not tightly controlled leading to variation and complexity in the response. The use of polydimethylsiloxane (PDMS) microfluidic channels provides a unique and controlled environment where the electric field is uniformly axial along the entire channel. Among other stimuli to control movement, food26 and UV light31 could equally be used but they are either temporally slow (food) or fatal (UV light) to the organism.
In this paper, we demonstrate for the first time that C. elegans exhibits directed movement inside microchannels in response to an electric field that is reliable and highly reproducible. We provide evidence that the electric field can be used as a powerful stimulus to efficiently control and orient worms as desired. We have also characterized the biological basis of this response in microchannels and show that it is primarily mediated by neuronal signals. Finally, we show that exposure to electric field is not harmful to worms since they continue to live normally and remain fertile. Our findings have the potential to use electric field-based microfluidic devices to study worm movement and to understand the genetic basis of electrotaxis. Additionally, these devices could also facilitate high throughput screening (HTS) of chemicals using movement as an assay that has historically not been possible with existing microfluidic formats. Among other applications, our work could also help to develop micro-worm sorters to separate different stages of animals for genetic experiments.
Device fabrication
The mask layout was designed in AutoCAD (Autodesk Inc., San Francisco, USA) and printed using ultra high-resolution laser photoplotting on transparency sheet. SU8-100 (80 µm thick) negative photoresist (MicroChem Corp., MA, USA) was used to lithographically pattern a master mold of our device. Polydimethylsiloxane (PDMS) pre-polymer mixture (Sylgard 184 kit, Dow Corning Corp., MI, USA; 10: 1 ratio of the base and cross-linker) was then cast on the master mold, and cured at room temperature for 24 h. The PDMS replica was then peeled off the master mold and cut into pieces containing individual channels. The inlet and outlet access ports were punched out at the reservoir areas. The top surface of the PDMS replica and a bare PDMS piece of the same size were plasma oxidized (50 W for 30 s), micro-contact printed with PDMS pre-polymer, and bonded, sealing the microchannel. Inlet and outlet capillary glass tube tips (VWR International, USA, catalog number CA14672-380, 1.5 mm outer diameter, 20 mm long) were connected to the punched areas. Plastic tubes (Saint-Gobain Performance Plastics, OH, USA, TYGON R-3603, 2.4 mm outer diameter and 10 cm long) were connected to the inlet and outlet glass tubes. Metal electrodes (Arcor Electronics, USA, C24, copper 0.5 mm diameter) were inserted into the reservoir areas by punching through the PDMS elastomer from the side. Liquid PDMS pre-polymer was then used to seal the surrounding areas of the electrodes and the device was placed on a hot plate (120 °C) to cure. The device was then attached to a glass cover slip again using PDMS pre-polymer and cured.
Electrotaxis test setup
The experimental setup to study nematodes' response to an electrical field (Fig. 1a) consists of four major parts: a microdevice (microchannel, length scale measurement setup, and electrodes at reservoirs), a worm-handling unit (syringe pump, inlet and outlet connections), actuation (power supply and electrodes), and a monitoring unit (microscope and camera). The microdevice consists of a simple microchannel (300 µm wide, 80 µm deep, and 5 cm long) instrumented with electrodes (5 cm apart) at reservoirs at both ends of the channel (Fig. 1b).
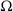 . The videos were recorded to obtain raw data presented in this paper.
. The videos were recorded to obtain raw data presented in this paper.
Nematode strains and culturing
Worms were grown at room temperature (20 °C) on standard NG agar plates seeded with OP50 Escherichia coli bacteria as previously described.32 The strains used in this study are: N2, BC347 unc-54(s74), CB78 unc-6(e78), PS55 lon-2(e678), and PS250 dpy-5(e61). The PS55 strain also carries a him-5(e1490) mutation that increases frequency of males in the progeny. The N2 strain was used as a wild-type reference in all assays.
Nearly all experiments were done with synchronized stages of animals. Gravid hermaphrodites were washed off culture plates using M9 buffer (3 g KH2PO4, 6 g Na2HPO4, 5 g NaCl, and 1 mL 1M MgSO4 in 1 L). They were centrifuged and washed twice with M9 to remove excess bacteria and debris. A 2 mL of bleach solution (800 µL of 4 N NaOH and 1200 µL of commercial bleach) was added to 4 mL of worms. The mixture was incubated at room temperature for 3 min and then centrifuged and washed with M9 (at least 3×). The eggs were allowed to hatch in M9 for 24 h. They were subsequently transferred to NG agar plates seeded with OP50 bacteria and were allowed to grow further. When required for testing, the worms were washed again with M9 and loaded in microchannels.
For electrokinetic flow tests dead animals were obtained as below. Wild type C. elegans were synchronized using the above bleach protocol and kept in M9. The animals were left in the absence of food for one week at room temperature causing them to die. The dead animals appeared rod-shaped with no visible body bending or movement.
Data analysis
A length measurement scale was microfabricated alongside the microchannel as shown in Fig. 1b and 2. The response of worms to different ranges of electric fields was recorded by a camera (Nikon Coolpix P5100, NY, USA) and analyzed by ImageJ software (http://rsbweb.nih.gov/ij/) and AutoCAD (http://www.autodesk.com). ImageJ was used to analyze videos and obtain the snapshots (every 0.07 s) of recorded movies of worm movements. The sequenced images were used to measure the distance traveled by the worm inside the channel. For this purpose, the initial and final snapshots were imported into AutoCAD and the traveled length was measured by superimposing lines on the worms' pathway and comparing the line lengths to a reference length bar in the image. Snapshots obtained from ImageJ were used to measure the worm movement distance between length scales fabricated on the device. The worms' lengths were also measured by linear approximation method using AutoCAD software. One image of each tested worm was imported to the software. A total of 15 lines were drawn on the worms' body image. The lengths of the lines were added and the total length was compared to the reference value to determine each worm's length. This process was repeated three times for each worm and an accuracy of ~10 µm was obtained.
Movement and electrotaxis of C. elegans in microchannels
Previous studies reported that C. elegans respond to electrical signals on open agar gel surfaces and move towards the negative pole.28,29 It was shown that this behavior is mediated by certain amphid sensory neurons that are sensitive to direction and strength of the electrical signal.29 These studies focused on only adult worms and showed that the animals moved at an angle towards the negative pole. Furthermore, it was shown that the angle of their path increased with the magnitude of the electric field. The microchannel format, as it is used here, allows electric streamlines to be confined and directed along the axis of the channel and provides a simple well-controlled format to study and understand the electrotaxis of C. elegans.
The experimental setup used in this study is shown in Fig. 1a. Straight microchannels (5 cm long and 80 µm deep) with varying widths of 2 mm, 1 mm, 500 µm, 300 µm, and 150 µm with electrodes instrumented in their reservoirs (Fig. 1b) were fabricated using soft lithography method33 (see Experimental section for fabrication process). Synchronized C. elegans (see Experimental section) of various age and size, from L1 (~250 µm long) to young adult (~1 mm long), were loaded individually into the microchannels filled with M9 buffer and positioned in the central section (2.5 cm away from each electrode) using a syringe pump. In the absence of a stimulus, the animals had random movement in the microchannel (n = 20). In some instances, we observed that after traveling a certain distance in one direction, animals turned and moved in the opposite direction. In two cases animals exited the channel after spending 5 min inside the channel. The microdevice with 300 µm wide microchannel appeared to be the most optimum in our assays because it guided movement along the channel axis without any obvious physical confinement and eliminated any perpendicular motion of the worms. The 150 µm wide microchannel was too narrow and appeared to interfere with worms' normal swimming behavior and hence was not used.34
The above setup allowed us to examine the response of worms to an electric field. We found that in the presence of low-voltage electric fields, worms exhibited electrotaxis and moved in a directed manner towards the negative pole. To rule out the influence of electrokinetic flows (electrophoresis as well as electro-osmosis), dead worms (see Experimental section) were loaded individually into the channels filled with M9 solution and positioned in the middle section (2.5 cm away from the electrodes) using a syringe pump. A wide range of electric field strengths (1–20 V cm−1) was applied across the channel that showed that electrokinetic flow above 13 V cm−1 was able to move dead worms towards the anode. No change in the morphology of dead worms was observed during this process. Furthermore, we found that the electrokinetic effects (electrophoresis of the worm and electro-osmosis of fluid) had no significant role in the movement of worms below 13 V cm−1 in these confined geometries.
To characterize the electrotactic behavior in more detail, synchronized animals of different stages (from L1 to young adult) were introduced into the microchannel. A wide range of electric fields (1–12 V cm−1) was applied across the channel and movement of animals was monitored. We observed that early stage animals (L1 and L2) displayed no obvious response to the feasible electric field (1–12 V cm−1) since they continued to swim randomly regardless of the direction and presence of the field. At later stages (L3 onwards), animals responded robustly to the electric field within a certain range that was different for each stage and exhibited directed movement towards the negative pole [see supplementary movie 1, ESI![]() ]. A careful examination revealed that their swimming pattern was typical of unexposed animals in a liquid environment except that the response was directional. This suggests that the electric field does not distort body bends rather it simply induces swimming behavior. Below the minimum threshold, the movement was found to be random whereas above the maximum threshold, worms appeared paralyzed although they resumed swimming upon the removal of the electric field suggesting that the effect was reversible. Using a length scale, fabricated alongside the microchannel (Fig. 1b), we measured the speed under an effective electric field range of response. The finding that only older worms responded to the electric field suggests that this behavior is developmentally regulated and is likely to be mediated by certain differentiated cell types that may be absent (or immature) at earlier L1 and L2 stages. Other possibility could be that early stage animals respond to electric fields above 12 V cm−1 that is beyond the feasible range in our setup. These results demonstrate for the first time that older larvae and young adults of C. elegans respond to electric field in a liquid environment inside the microchannel and move in a directed manner.
]. A careful examination revealed that their swimming pattern was typical of unexposed animals in a liquid environment except that the response was directional. This suggests that the electric field does not distort body bends rather it simply induces swimming behavior. Below the minimum threshold, the movement was found to be random whereas above the maximum threshold, worms appeared paralyzed although they resumed swimming upon the removal of the electric field suggesting that the effect was reversible. Using a length scale, fabricated alongside the microchannel (Fig. 1b), we measured the speed under an effective electric field range of response. The finding that only older worms responded to the electric field suggests that this behavior is developmentally regulated and is likely to be mediated by certain differentiated cell types that may be absent (or immature) at earlier L1 and L2 stages. Other possibility could be that early stage animals respond to electric fields above 12 V cm−1 that is beyond the feasible range in our setup. These results demonstrate for the first time that older larvae and young adults of C. elegans respond to electric field in a liquid environment inside the microchannel and move in a directed manner.
Effect of age on electrotaxis
The developmental response of worms to the electric field stimulus led us to characterize it in further detail. Fig. 2 shows snapshots of typical electrotactic movements of C. elegans in the microchannel. At the minimum threshold electric field (2 V cm−1 for young adults), worms showed robust movement and oriented themselves towards the cathode [see supplementary movie 1, ESI![]() ]. We analyzed the movement of larvae and young adults (Fig. 3). The results showed that the speed of individual worms at any one particular stage does not change significantly with variations in the applied electric field; however, older worms had a higher speed when compared with the younger ones (~80% increase between L3 and L4, and ~35% increase between L4 and adult). We found that this difference in speed was effective in separating worms of two different stages (see Fig. 4 for L3 and young adult animals' separation).
]. We analyzed the movement of larvae and young adults (Fig. 3). The results showed that the speed of individual worms at any one particular stage does not change significantly with variations in the applied electric field; however, older worms had a higher speed when compared with the younger ones (~80% increase between L3 and L4, and ~35% increase between L4 and adult). We found that this difference in speed was effective in separating worms of two different stages (see Fig. 4 for L3 and young adult animals' separation).
The L3 stage animals responded to the electric field robustly starting from 4 V cm−1 (minimum threshold). The maximum threshold (defined by the paralysis phenotype) could not be observed because animals continued to swim normally without a change in speed even at the maximum allowable field (12 V cm−1 with no electrokinetic flow effect) in the channel. At later stages, animals appeared more sensitive to the electric field. Thus, while L4 stage animals were partially paralyzed at 10 V cm−1 (revealed by occasional abnormal body bends and reduced speed), the young adults exhibited this effect at 4 V cm−1. The minimum threshold response at these two stages was 4 V cm−1 (L4) and 2 V cm−1 (young adult). Reversing the applied electric field resulted in the reversal of the worm's movement. These results demonstrate that adult animals are more sensitive to the electric field and possess the shortest response range compared to L3 and L4 larvae.
We also studied the undulatory motion of worms of different sizes to determine the frequency of body bending under various electric fields using a no-field application as the witness model. It was observed that in the presence of electric field the average bending frequency of worms of different sizes (450–1000 µm long) (n = 15) ranged between 1.7 and 2.6 Hz. The bending frequency for each worm did not change significantly (<5% variation at maximum) with a change in the electric field strength or/and the direction and it was in close approximation of the no-field movement frequency (1.78–2.52 Hz for different size animals) demonstrating that the electric field had only a minor effect on the worm's natural body motion. These measurements compare favorably with other studies with C. elegans in microstructured environments34,35 where the bending frequency increased from 1.5 Hz on a gel surface to 1.92 Hz inside microchambers filled with micropillars.
Cellular basis of electric field responses in microchannels
We used mutant worms with defects in specific cell types to explore the cellular basis of electrotaxis. Gabel et al.29 had previously shown that the neuronal circuits of C. elegans, specifically the amphid sensory neurons, mediate electrosensory behavior. We carried out similar experiments using unc-6(e78) mutant animals that exhibit defects in neuronal differentiation. Studies on unc-6 have shown that it encodes a netrin-like secreted protein that plays a crucial role in neuronal growth cone migrations.36 The unc-6 mutant animals are uncoordinated due to defects in dorsal and ventral nerve cords.37 The analysis of unc-6(e78) young adults in the microchannel revealed no response to the electrical stimulus. The animals showed no obvious sign of orientation and speed change following the application of electric fields [see Fig. 5 and supplementary movie 2, ESI![]() ].
].
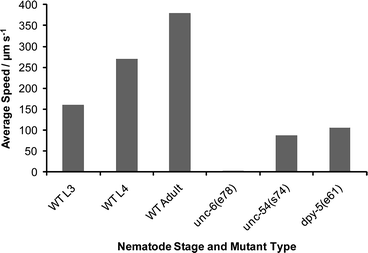
We also tested a muscle mutant unc-54(s74) in a similar setting to determine the contribution of muscles in electric field-driven swimming behavior. The unc-54 gene is necessary for the proper differentiation of the muscle myosin class II heavy chain (MHC B).38 The unc-54 mutant animals exhibit disorganized muscles and are severely uncoordinated (paralyzed).39 We found that unc-54(s74) young adult worms responded to the electric field in a manner similar to that of wild-type worms (2–4 V cm−1 range) although their speed was significantly slower (unc-54: 88 µm s−1, wild type: 380 µm s−1, average speeds) [see Fig. 5 and supplementary movie 3, ESI![]() ]. This is interesting considering that unc-54(s74) worms on standard culture plates (NG agar) exhibit almost complete paralysis. These results allow us to conclude that the electrosensory response in microchannels is primarily mediated by neuronal activity.
]. This is interesting considering that unc-54(s74) worms on standard culture plates (NG agar) exhibit almost complete paralysis. These results allow us to conclude that the electrosensory response in microchannels is primarily mediated by neuronal activity.
Sensitivity of electric field response: size vs. development
Our experiments have revealed that adult worms respond to electrical fields much more robustly compared to younger (L3 stage) animals (Fig. 3). Considering that electrosensory behavior is mediated by neurons, it is possible that adult worms have a mature nervous system and are therefore capable of processing neuronal signals more efficiently than the younger developing worms. Such a difference may also result from a change in the length of worms since adult worms are significantly larger compared to L3 stage animals (~1000 ± 100 µm and ~450 ± 100 µm, respectively). To distinguish between these possibilities, we tested two different mutant strains that are shorter and longer (dpy-5(e61) and lon-2(e678), respectively) compared to the wild type. The dpy-5 gene encodes a collagen that is necessary for the cuticle formation in developing larvae whereas lon-2 encodes a glypican family of heparan sulfate proteoglycans that negatively regulates DBL-1/BMP signaling to control body length.40,41 Mutations in these two genes give rise to opposite phenotypes. Thus while dpy-5 mutant animals are approximately 60% shorter compared to the wild type (400 ± 100 µm and 1000 ± 100 µm at 62 h, respectively), the lon-2 mutant animals are roughly 30% longer (1300 µm ± 100 µm at 62 h) than wild type. Both these mutant animals are otherwise healthy and active.
The analysis of dpy-5(e61) animals in the microchannel revealed that, unlike wild type animals (effective response range of 2 to 4 V cm−1), these animals responded to the electric field robustly starting from 4 V cm−1 and showed no sign of paralysis at the highest possible field tested (12 V cm−1). Their average speed (106 µm s−1) did not change in response to an alteration in the electric field strength and/or direction. In contrast, the lon-2(e678) animals did respond to the lowest threshold electric field as the wild type (2 V cm−1). However, these animals appeared extremely sensitive and were paralyzed under the influence of electric fields higher than 3 V cm−1. Due to their larger size, they were unable to move freely in the microchannel and exhibited abnormal movements. This precluded us from measuring their speed. These results demonstrate that longer worms are more sensitive to the electric field than the shorter worms suggesting that the size is a major determinant of the sensitivity of C. elegans to the electric field. This was most likely due to differences in the potential drop across the entire body that is greater in lon mutants compared to the wild type (~30%). This may also explain the variability in responses observed for different stages of wild type animals since they are not exactly alike.
Post-exposure effect of electric field
Considering that worms appeared paralyzed when exposed to electric fields greater than their response range, we determined the post-exposure effect in our assays by examining their behavior, fertility and viability. For this, 10 adult animals were aspired into the channel individually and a constant electric field (2–4 V cm−1) was applied across the channel for a duration of 10 min in each case. During this period, the polarity of the field was reversed every minute (while keeping the field strength constant) in order to keep the worm inside the channel and to prevent it from getting in direct contact with electrodes. In one case we also exposed a young adult worm to 12 V cm−1 electric field (three times that of maximum threshold electric field of wild type animals) for a duration of 10 min. Following the exposure, worms were removed and grown on standard culture plates. We found that in all cases (n = 11), animals recovered successfully within few hours, exhibited normal sinusoidal pattern of movement (i.e., no uncoordinated movement), did not die prematurely (~18 days average age), and were fertile for 3–4 days (similar to unexposed wild type worms). This demonstrates that the electric field stimulus causes no visible harm to C. elegans and that there are no apparent long-term developmental and behavioral changes following exposure. However, we cannot rule out the possibility of certain cellular and molecular changes that could have occurred. This will require more extensive analysis using molecular markers and ultra-structural studies.
This study focuses on the C. elegans behavior in a microfluidic environment and demonstrates that a low-voltage electric field could be used as an attractant to guide their movement without physiological and behavioral side effects. We have shown that the exposure of electric field to worms induces forward movement towards the cathode that is robust, highly reproducible, and sensitive.
We analyzed the electric field response in microchannels and identified a range of electric field strengths (with minimum and maximum thresholds) within which an optimum electrotactic response is observed. Not all stages of animals responded equally well within the same threshold range. Thus while the effective range for L4 larvae was 4–10 V cm−1, the adults appeared significantly more sensitive and had a lower response threshold (2–4 V cm−1). Within the optimum range at any given stage, the speed of movement of animals remained unchanged suggesting that the electric field response is a binary phenomenon (all or none). All responding stages of animals (L3, L4, and young adult), when exposed to the electric field above the maximum threshold, exhibited paralysis as judged by their near rod-like shape and abnormal body bends. This effect was reversible since the animals resumed normal movement upon lowering the electric field. Furthermore, we found that the electric field manipulation of C. elegans is not harmful in an obvious manner since the exposed animals, when placed on standard culture plates, resumed normal movement and feeding behavior and continued to reproduce normally.
Our findings that adult animals are more sensitive to the electric field compared to the larvae led us to examine this issue in some detail. We considered length being a factor since the adult worm is much larger than larvae (up to ~50% in the case of young adult and L3 larvae) and furthermore longer objects are expected to experience more potential drop overall compared to the shorter ones. In agreement with this, our experiments revealed that the sensitivity to electric field indeed depends on the length of animals. Using mutant animals that are longer (~30%, lon-2 mutants) and shorter (~60%, dpy-5 mutants) compared to the wild type, we found that sensitivity to the electric field is directly proportional to the length of animals.
We examined the cellular basis of electrotaxis in a liquid environment and found that neurons, are necessary to induce this behavior. This is consistent with the finding of Gabel et al.29 and suggests that electrical cues are likely to be processed by the same set of neurons and genetic pathway regardless of the environment of animals. Furthermore, our work has also uncovered the importance of muscles in mediating electrotaxis in a microfluidic environment. Unlike previously described pneumatic microdevices that rely on forced liquid flow to move worms, the use of the electric field stimulus in our assay appears to induce a precise and sensitive innate movement response. Therefore, it could be potentially used in movement-based behavioral HTS assays for drugs/chemicals in a microfluidic format. For example, a combination of null mutations in dystrophin (dys-1) and MyoD (hlh-1) genes in C. elegans has been shown to cause progressive muscle degeneration similar to human DMD (Duchenne's muscular dystrophy) that impairs movement.8,42–44 The electric field-based microfluidic channel may facilitate HTS of drugs that improve/restore movement, thereby identifying potential candidates to test in human DMD patients. Additionally, our assay setup could also be used to study the mechanism of electrotaxis as well as how nervous system processes extracellular signals in general. Because movement is a complex behavior that is controlled by many genes, our findings promise the design of better microfluidic-based assays to study the function of genes and pathways that mediate this behavior.
This work was supported financially from the Canada Research Chairs program and McMaster University to BPG, from NSERC and OCE to PRS, and from NSERC summer scholarship to AS.
| 1 | O. Hobert, Specification of the nervous system (August 8, 2005), in WormBook, ed. The C. elegans Research Community, WormBook, , DOI:10.1895/wormbook.1.12.1, http://www.wormbook.org |
|
| 2 | E. Denayer, T. de Ravel and E. Legius, J. Med. Genet., 2008, 45, 695–703. | |
| 3 | N. Moghal and P. W. Sternberg, Exp. Cell Res., 2003, 284, 150–159. | |
| 4 | T. Kaletta and M. O. Hengartner, Nat. Rev. Drug Discovery, 2006, 5, 387–398. | |
| 5 | R. Baumeister and L. Ge, Trends Biotechnol., 2002, 20, 147–148. | |
| 6 | K. Ashrafi, F. Y. Chang, J. L. Watts, A. G. Fraser, R. S. Kamath, J. Ahringer and G. Ruvkun, Nature, 2003, 421, 268–272. | |
| 7 | T. C. Kwok, N. Ricker, R. Fraser, A. W. Chan, A. Burns, E. F. Stanley, P. McCourt, S. R. Cutler and P. J. Roy, Nature, 2006, 441, 91–95. | |
| 8 | L. Segalat, ACS Chem. Biol., 2007, 2, 231–236. | |
| 9 | C. Voisine and A. C. Hart, Methods Mol. Biol. (Totowa, N. J.), 2004, 277, 141–160 |
|
| 10 | C. Voisine, H. Varma, N. Walker, E. A. Bates, B. R. Stockwell and A. C. Hart, PLoS One, 2007, 2, e504. | |
| 11 | M. Lakso, S. Vartiainen, A. M. Moilanen, J. Sirvio, J. H. Thomas, R. Nass, R. D. Blakely and G. Wong, J. Neurochem., 2003, 86, 165–172. | |
| 12 | L. Segalat, ACS Chem. Biol., 2006, 1, 277–278. | |
| 13 | M. Artal-Sanz, L. de Jong and N. Tavernarakis, Biotechnol. J., 2006, 1, 1405–1418 |
|
| 14 | C. J. Locke, S. A. Fox, G. A. Caldwell and K. A. Caldwell, Neurosci. Lett., 2008, 439, 129–133. | |
| 15 | S. Lockery, Nat. Methods, 2007, 4, 691–692. | |
| 16 | S. E. Hulme, S. S. Shevkoplyas and A. Samuel, Nat. Methods, 2008, 5, 589–590. | |
| 17 | N. Kima, C. M. Dempseyc, J. V. Zoval, J. Sze and M. J. Madou, Sens. Actuators, B, 2007, 122, 511–518. | |
| 18 | C. B. Rohde, F. Zeng, R. Gonzalez-Rubio, M. Angel and M. F. Yanik, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 13891–13895. | |
| 19 | K. Chung, M. M. Crane and H. Lu, Nat. Methods, 2008, 5, 637–643. | |
| 20 | F. Zeng, C. B. Rohde and M. F. Yanik, Lab Chip, 2008, 8, 653–656. | |
| 21 | S. X. Guo, F. Bourgeois, T. Chokshi, N. J. Durr, M. A. Hilliard, N. Chronis and A. Ben-Yakar, Nat. Methods, 2008, 5, 531–533. | |
| 22 | A. Ben-Yakar and F. Bourgeois, Curr. Opin. Biotechnol, 2009, 20, 100–105. | |
| 23 | S. E. Hulme, S. S. Shevkoplyas, J. Apfeld, W. Fontana and G. M. Whitesides, Lab Chip, 2007, 7, 1515–1523. | |
| 24 | J. X. Zhang, HSFP J, 2007, 1, 220–224 |
|
| 25 | N. Chronis, M. Zimmer and C. I. Bargmann, Nat. Methods, 2007, 4, 727–731. | |
| 26 | J. Qin and A. R. Wheeler, Lab Chip, 2007, 7, 186–192. | |
| 27 | R. Gaugler and A. L. Bilgrami, Nematode behaviour, CABI Pub., Wallingford, Oxfordshire, Cambridge, MA, 2004 |
|
| 28 | N. C. Sukul and N. A. Croll, J. Nematol., 1978, 10, 314–317 |
|
| 29 | C. V. Gabel, H. Gabel, D. Pavlichin, A. Kao, D. A. Clark and A. D. Samuel, J. Neurosci., 2007, 27, 7586–7596. | |
| 30 | D. R. Viglierchio and P. K. Yu, Rev. Nematol., 1983, 6, 171–178 |
|
| 31 | A. Ward, J. Liu, Z. Feng and X. Z. Xu, Nat. Neurosci., 2008, 11, 916–922. | |
| 32 | S. Brenner, Genetics, 1974, 77, 71–94. | |
| 33 | Y. Xia and G. M. Whitesides, Annu. Rev. Mater. Sci., 1998, 28, 153–184. | |
| 34 | S. R. Lockery, K. J. Lawton, J. C. Doll, S. Faumont, S. M. Coulthard, T. R. Thiele, N. Chronis, K. E. McCormick, M. B. Goodman and B. L. Pruitt, J. Neurophysiol., 2008, 99, 3136–3143. | |
| 35 | S. Park, H. Hwang, S. W. Nam, F. Martinez, R. H. Austin and W. S. Ryu, PLoS One, 2008, 3, e2550. | |
| 36 | N. Ishii, W. G. Wadsworth, B. D. Stern, J. G. Culotti and E. M. Hedgecock, Neuron, 1992, 9, 873–881. | |
| 37 | E. M. Hedgecock, J. G. Culotti and D. H. Hall, Neuron, 1990, 4, 61–85. | |
| 38 | A. R. MacLeod, J. Karn and S. Brenner, Nature, 1981, 291, 386–390. | |
| 39 | D. G. Moerman, S. Plurad, R. H. Waterston and D. L. Baillie, Cell, 1982, 29, 773–781. | |
| 40 | T. L. Gumienny, L. T. MacNeil, H. Wang, M. de Bono, J. L. Wrana and R. W. Padgett, Curr. Biol., 2007, 17, 159–164. | |
| 41 | C. Thacker, J. A. Sheps and A. M. Rose, Cell. Mol. Life Sci., 2006, 63, 1193–1204. | |
| 42 | J. S. Chamberlain and G. M. Benian, Curr. Biol., 2000, 10, R795–797. | |
| 43 | K. Grisoni, K. Gieseler, M. C. Mariol, E. Martin, M. Carre-Pierrat, G. Moulder, R. Barstead and L. Segalat, J. Mol. Biol., 2003, 332, 1037–1046. | |
| 44 | A. Gaud, J. M. Simon, T. Witzel, M. Carre-Pierrat, C. G. Wermuth and L. Segalat, Neuromuscul. Disord., 2004, 14, 365–370. |
Footnote |
|
|
| This journal is © The Royal Society of Chemistry 2009 |
FIGURES
Fig. 1 The schematics of the experimental setup. The four major parts consist of (a) a worm handling unit (syringe pump, sample container, inlet, and outlet pipes), a monitoring unit (digital camera and microscope lenses), an actuation unit (power source and electrodes), and (b) the microchannel device (sealed PDMS microchannel with embedded electrodes in reservoir areas).
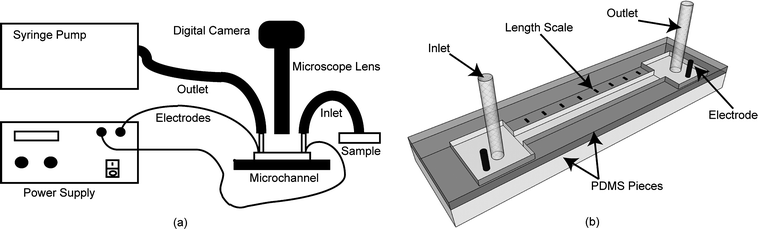
Fig. 2 The movement of worms in an electric field. (a) The application of +8 V cm−1 electric field (E) caused an animal (724 µm long) to move with the speed of 308 µm s−1 to the right towards the cathode. (b) At a lower field strength in a reverse direction (−3 V cm−1) the animal (847.5 µm long) moved with a speed of 342 µm s−1 to the left towards the cathode. Dark thick arrows illustrate the worm's position. Scale bars are 1 mm.
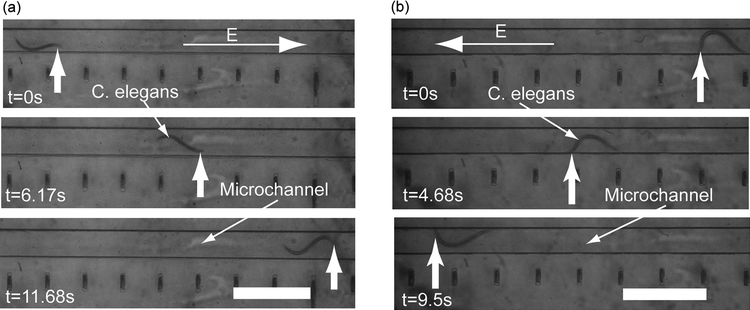
Fig. 3 The effect of electric field on different stages of C. elegans. The L3 stage worms (385–528 µm long, dark rectangles) responded to electric fields above 4 V cm−1 with a speed range of 100 to 216 µm s−1. The L4 stage worms (534–725 µm long, clear rhombuses) responded to electric fields between 4 and 10 V cm−1 with a speed range of 220 to 340 µm s−1. Due to the partial paralysis at 12 V cm−1, the speed of L4 stage worms was reduced. The young adults (920–1050 µm long, dark circles) had the lowest effective electric field range (2–4 V cm−1) since they were paralyzed above 4 V cm−1. Within the effective range, their speed ranged between 296 and 471 µm s−1. The upper threshold electric field was not observed for L3 stage worms due to the upper limit of allowable field without electrokinetic flow.
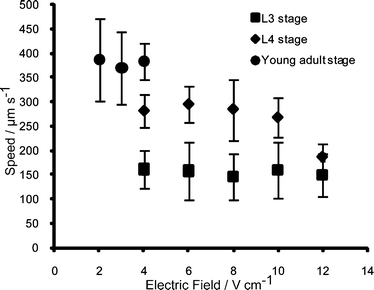
Fig. 4 Separation of two animals (530 µm long L3 stage and 1000 µm long young adult) within 6 s upon application of 4 V cm−1 electric field. The thin and thick white arrows mark the anterior ends of the L3 stage and young adult worms, respectively. The scale bar is 1 mm.
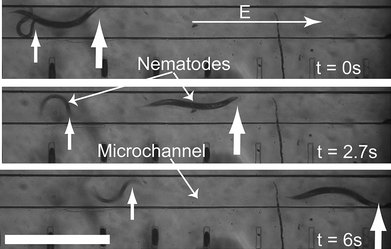
Fig. 5 Average speed of various animals in a 5 cm long, 300 µm wide and 80 µm deep microchannel. WT, Wild type.